New technique may help quicken production of blood cancer drug
Redistributed by neucrad health & written by
Sunderarajan Padmanabhan New Delhi, Monday, February 11
Researchers at Madurai Kamaraj University have developed a technique that promises to quicken the process of production of L-asparaginase, an enzyme used in treatment of acute lymphoblastic leukaemia.
Human cells need steady supply of an amino acid, Asparagine, to build proteins and they use an enzyme called asparagine synthetase to make it. Cancerous cells, however, rely on blood for their requirement of asparagine.
L-Asparaginase therapy takes advantage of this. L-Asparaginase enzyme performs the opposite reaction to asparagine synthetase. It catalyzes conversion of L-asparagine to aspartic acid and ammonia. If a large dose of this enzyme is introduced into the blood, it will circulate and continually break down all asparagine that it finds, ultimately starving the cells that depend on blood for asparagine supply.
L-Asparaginase is an effective therapy for those cases where blood cells become cancerous, such as in acute lymphoblastic leukemia. L-Asparaginase cuts off the supply of asparagine in blood and cancer cells die as they are unable to build their proteins. It is produced from various microbes including a marine bacterium called Bacillus tequilensis PV9W.
“L-Asparaginase is an effective therapy for those cases where blood cells become cancerous, such as in acute lymphoblastic leukemia. ”

Researchers cloned the gene coding for L-asparaginase from Bacillus tequilensis PV9W and expressed it in Escherichia coli BL21 bacterium. In just 12 hours they could produce as much quantity of recombinant enzyme as the native Bacillus tequilensis PV9W could do in 48 hours. “We have been able to cut down the production time by 75 per cent at the laboratory scale. It should be possible to extrapolate the process at commercial scale,” explained Dr. P. Varalakshmi, leader of the research team.
The team has also developed another technique that promises to improve shelf life of the L-asparaginase enzyme and enhance its activity. Researchers encapsulated native L-asparaginase of Bacillus tequilensis PV9W using solid lipid particles and hot lipid emulsion. “Tests showed that the encapsulated enzyme was stable for 25 days even when stored at 25 degrees as compared to the native enzyme which needed refrigerated conditions,” researchers said.
The enzyme has been tested on cervical cancer cell line. “The cytotoxicity ability of the particle was found to be highly enhanced compared to the native L-asparaginase from Bacillus tequilensis PV9W,” they said.
In addition, the team has found that lipid encapsulated enzyme could be used to detect L-asparagine in cell extracts using electrochemistry method, Differential Pulse Voltammetry (DPV). This work was done in collaboration with researchers at Central Electro Chemical Research Institute (CECRI) at Karaikudi. Researchers coated a glassy carbon electrode with the lipid encapsulated enzyme and found that there was a difference in the generated current when the electrode was in contact with L- asparagine. This means lipid encapsulated enzyme could possibly be used as a biosensor to monitor the cancer progression and treatment by L-asparaginase.
Besides Dr. P. Varalakshmi, the research team included Senior Scientist Dr. V. Ganesh of CECRI, Prof. Dr.V.S.Vasantha of School of Chemistry at MKU, Dr. B. Ashok Kumar, School of Genetic Engineering, MKU and research scholars Dr.G. Shakambari, and Mr. Sameer Kumar Rai. The findings have been published in journal Scientific Reports.
Image and news source: India Science wire
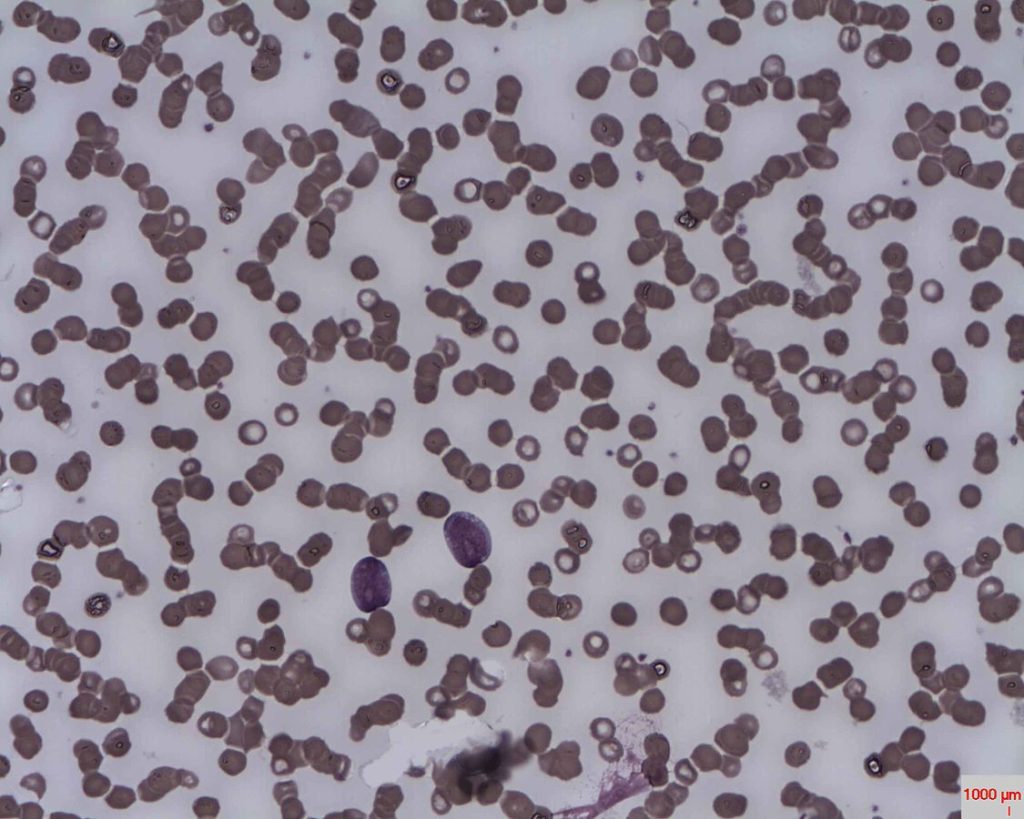
About the featured Image and credit: Peripheral blood film in Acute Lymphoblastic Leukaemia (ALL) for illustration purpose only. Author:
Dr Erhabor Osaro, CC-BY-SA-3.0