Anti-Psoriasis Drug Itolizumab is Approved in India to Treat COVID-19 Following Human Trial with 30 Subjects
Dr. Shuvomoy Banerjee, Neucrad Health Desk, July 18, 2020
Biocon, a Bangalore-based biopharmaceutical company, has received approval for its plaque psoriasis drug Itolizumab (Brand name- Alzumab) for emergency use in Covid-19 patients from the Drugs Controller General of India (DCGI) following human trial with 30 subjects.
The approval is to treat cytokine release syndrome (CRS) having moderate to severe acute respiratory distress syndrome (ARDS) with Covid-19 patients.
Biocon and the Cuban Center for Molecular Immunology (CIM) conducted a human trial on novel coronavirus infected patients using a repurposed drug called Itolizumab. In 2013, Biocon already received DCGI approval for the use of Itolizumab to treat psoriasis (An inflammatory, autoimmune disease). However, in May of this year, DCGI has again approved and suggested this anti- psoriasis drug for human trial with Covid-19 patients. Therefore, Biocon has conducted the trial on a total of 30 corona patients from different hospitals in Delhi, Bangalore and Mumbai. In this trial, 20 patients were treated with other antivirals, antibiotics, hydroxy-chloroquine, and oxygen (called ‘supportive care’) along with the Itolizumab. The remaining 10 patients received the best supportive care except itolizumab. The test showed that only 20 patients undergoing itolizumab treatment could completely recover. This important observation and result encouraged scientists to know more about the drug repurposing strategies and underlying molecular mechanism for the therapeutic intervention of Covid-19.
What is Itolizumab and how does it work?
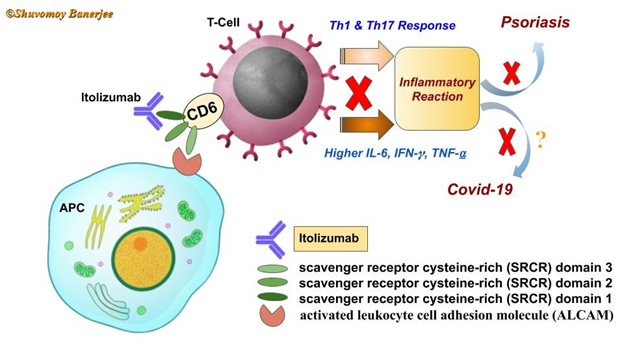
Itolizumab is a humanized monoclonal antibody (IgG1) that binds with ‘co-stimulator’ glycoprotein molecule CD6 (cluster of differentiation 6) located in the cell membrane of T-lymphocytes. According to the biochemical structure, CD6 has three scavenger receptor cytosine-rich (SRCR) protein domains. Researchers demonstrated that CD6 ligands are commonly found in B-lymphocytes, dendritic cells and antigen-presenting cells (APCs). The specific ligand is called CD166 or Activated Leukocyte Cell Adhesion Molecule (ALCAM). ALCAM generally associate with SRCR-3 domain of CD6. The formation of CD6-ALCAM molecular complex stimulates T-lymphocytes and triggers the Th1, Th17 immune response. At the same time, the amount of various pro-inflammatory cytokines (IFN-6, IL-6, TNF-6) increases in the body. This leads to psoriasis, an inflammatory autoimmune disorder found in the skin. Interestingly, in Covid-19 situation, the amount of different cytokines in the body increases at an abnormal rate and a ‘cytokine storm’ is formed which damages various organs of the body.
Scientific research showed that Itolizumab binds to the SRCR-1 domain of CD6 and reduces the cell division rate of T-lymphocytes. Of Note, in T-lymphocytes, Itolizumab helps to decrease the amount of pro-inflammatory cytokines by regulating the function of some intracellular proteins (associated with CD6 mediated signaling pathways). Scientists speculate that Itolizumab which has far lower side effects than any other anti-inflammatory drugs may help to recover the Covid-19 patients by inhibiting override immune response.
Biocon Executive Chairperson Kiran Mazumdar-Shaw stated that the use of the drug has significantly reduced the mortality rate of Covid-19 patients. Biocon research partner, CIM in Cuba, used Itolizumab previously and got impressive results. Their tests showed that 79.2 percent of patients returned from the ICU and completely recovered after the treatment. According to the Biocon Chairperson, Itolizumab will do a great job for those SARS-CoV-2 infected patients particularly show the symptoms of Acute Respiratory Distress Syndrome (ARDS). Surprisingly, human trials have also indicated that the use of Itolizumab significantly increased the level of oxygen in the patient’s blood. She also informed regarding the treatment package cost. Usually, Covid-19 treatment requires a total of four doses of intravenous injection of Itolizumab. According to the company, each Itolizumab vial costs 8,000 rupees. They consider it’s a reasonable investment for this effective drug as it can reduce the expense for staying long in ICU and ventilation.
However, some suggestions have come from different doctors and scientists regarding this human trial. First, the full test was performed on a very small number of patients. In a very large group, there may be room for doubt as to whether the tests performed on a large number of patients would yield the same expected results or not.
Also, according to Dr. Varun Cheruparambat, the renowned heart specialist at the Medical Trust Hospital in Cochin has raised the concerning point that none of the patients who undergone Itolizumab treatment had to be given ventilation. Dr. Biswarup Ghosh, Biochemist & Neuroscientist, from US also expressed his concern about the approval of its emergency use.
“There are emerging evidences that COVID-19 induces diverse effects on different organs including but not limited to kidney, Heart, and Brain with preexisting diseases. Is the study on 20 human subjects on experimental group sufficient to measure the side effects and efficacy with the different stratification including age, sex, comorbidity status, etc ?” said Dr. Ghosh.
However, many in the small control group were on ventilation support. It is possible that there may be a slight base-line difference between the two groups in terms of age and co-morbidity. However, Biocon Chairperson said that the use of Itolizumab was allowed and applied in the most urgent novel corona situation considering the patient’s critical condition. In the near future, their published research papers will contain details of the full extended test.

References:
- Bughani U, Saha A, Kuriakose A, et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab [published correction appears in PLoS One. 2018 Jan 30;13(1):e0192335]. PLoS One. 2017;12(7):e0180088. Published 2017 Jul 3. doi:10.1371/journal.pone.0180088
- Srivastava A. Itolizumab in Psoriasis. Indian J Dermatol. 2017;62(4):418-421. doi:10.4103/ijd.IJD_467_16
- https://www.biocon.com/biocon_products_bio_BF_alzumab_pa.asp
- https://www.biocon.com/biocon_press_release_20200711.asp
- https://apps.who.int/trialsearch/Trial2.aspx?TrialID=RPCEC00000311
- Dogra S, Uprety S, Suresh SH. Expert Opin Biol Ther. 2017 Mar; 17(3):395-402. Epub 2017 Jan 25.
- Pai G, Pai AH. Case Rep Dermatol. 2017 May-Aug; 9(2):141-145. Epub 2017 Aug 23.
- Budamakuntla L, Shree-Lakshmi HV, Bansal A, Venkatarayaraju SK. Psoriasis (Auckl). 2019; 9:19-27. Epub 2019 May 3.







