New Animal Study Adds to Evidence of Parkinson’s Disease Origins in the Gut
Reading Time: 4 minutesExperiments in mice show transmission of nerve-killing protein from the gut into the brain
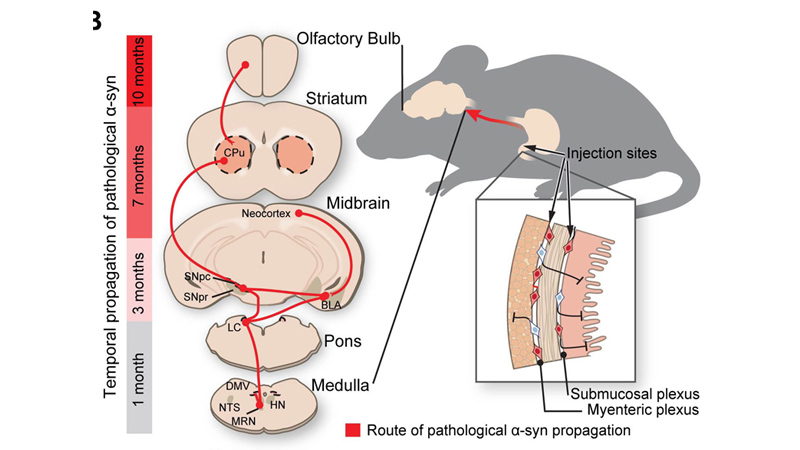
Route of Parkinson’s disease-causing protein propogation in mice. Credit: Ted Dawson
Neucrad Health India June 27, 2019
Source and credit: John Hopkins Medicine
In experiments in mice, Johns Hopkins Medicine researchers say they have found additional evidence that Parkinson’s disease originates among cells in the gut and travels up the body’s neurons to the brain. The study, described in the June issue of the journal Neuron, offers a new, more accurate model in which to test treatments that could prevent or halt Parkinson’s disease progression.
“These findings provide further proof of the gut’s role in Parkinson’s disease, and give us a model to study the disease’s progression from the start,” says Ted Dawson, M.D., Ph.D., director of the Johns Hopkins Institute for Cell Engineering and professor of neurology at the Johns Hopkins University School of Medicine. Parkinson’s disease is characterized by the buildup of a misfolded protein, called alpha-synuclein, in the cells of the brain. As more of these proteins begin to clump together, they cause nerve tissues to die off, leaving behind large swaths of dead brain matter known as Lewy bodies. As brain cells die, they impair a person’s ability to move, think or regulate emotions.
The new study builds off observations made in 2003 by German neuroanatomist Heiko Braak that showed people with Parkinson’s disease also had accumulations of the misfolded alpha-synuclein protein in the parts of the central nervous system that control the gut. The appearance of these neuron-damaging proteins is consistent with some early symptoms of Parkinson’s disease, which include constipation, says Hanseok Ko, Ph.D., associate professor of neurology at the Johns Hopkins University School of Medicine. Braak hypothesized that Parkinson’s disease advanced up the nerves connecting the gut and the brain like going up a ladder.
A growing body of evidence has implicated the gut-brain connection in initiating Parkinson’s disease. The researchers were most curious whether the misfolded alpha-synuclein protein could travel along the nerve bundle known as the vagus nerve, which runs like an electrical cable from the stomach and small intestine into the base of the brain.
To test this, the researchers injected 25 micrograms of synthetic misfolded alpha-synuclein created in the lab into the guts of dozens of healthy mice. The researchers sampled and analyzed the mouse brain tissue at one, three, seven and 10 months after injection. Over the course of the 10 month experiment, the researchers saw evidence that the alpha-synuclein began building where the vagus nerve connected to the gut and continued to spread through all parts of the brain.
The researchers then conducted a similar experiment, but this time surgically cut the vagus nerve in one group of mice and injected their guts with the misfolded alpha-synuclein. Upon examination at seven months, the researchers found that mice with severed vagus nerves showed none of the signs of cell death found in mice with intact vagus nerves. The severed nerve appeared to halt the misfolded protein’s advances, says Dawson.
The researchers then investigated whether these physical differences in Parkinson’s disease progression resulted in behavioral changes. To do this, they evaluated the behavior of three groups: mice injected with misfolded alpha-synuclein, mice injected with misfolded alpha-synuclein with cut vagus nerves, and control mice with no injection and intact vagus nerves. The researchers looked at tasks they commonly used to distinguish signs of mouse Parkinson’s disease, including nest building and exploring new environments.
The researchers first observed the mice build nests in their enclosure as a test for fine motor dexterity, which is commonly affected by Parkinson’s disease in humans. Healthy mice often make large, dense mounds in which to burrow. Smaller, messier nests are often signs of problems with motor control.
Seven months after injection, the researchers provided the mice with nesting materials and observed their nest building behavior for 16 hours, scoring their capabilities on a scale of 0–6. They found that mice that received the misfolded alpha-synuclein injection scored consistently lower on nest building.
While the control and severed vagus nerve groups consistently scored 3 or 4 on the nest building scale, mice that received the misfolded alpha-synuclein scored lower than 1. Also, while most mice used the entire 2.5 grams of material provided, the group of mice that received the alpha-synuclein injection used less than half a gram of the nesting material. In ways similar to Parkinson’s disease symptoms in humans, the mice’s fine motor control deteriorated as the disease progressed, says Ko.
In another experiment analyzing the mice for symptoms similar to Parkinson’s disease in humans, the researchers measured anxiety levels of the mice by monitoring how they responded to new environments.
For this test, the researchers placed the mice in a large open box where a camera could track their exploration. Healthy mice are curious and will spend time investigating every part of a new environment. However, mice affected by cognitive decline are more anxious, causing them to be more likely to stay toward the sheltered edges of a box.
The research team found that control mice and mice that had their vagus nerves cut to protect against Parkinson’s disease spent between 20 and 30 minutes exploring the center of the box. On the other hand, mice that received the misfolded alpha-synuclein injection but had intact vagus nerves spent less than five minutes exploring the center of the box and moved mostly around the borders, indicating higher anxiety levels, which the researchers report are consistent with symptoms of Parkinson’s disease.
Overall, the results of this study show that misfolded alpha-synuclein can be transmitted from the gut to the brain in mice along the vagus nerve, and blocking the transmission route could be key to preventing the physical and cognitive manifestations of Parkinson’s disease.
“This is an exciting discovery for the field and presents a target for early intervention in the disease,” says Dawson.
Next, the researchers say, they plan to explore what parts of the vagus nerve allow the misfolded protein to climb to the brain, and to investigate potential mechanisms to stop it.
Other researchers involved in the study include Sangjune Kim,
Seung-Hwan Kwon, Seung Pil Yun and Saebom Lee of the Johns Hopkins
University School of Medicine and the Adrienne Helis Malvin Medical
Research Foundation; Tae-In Kam, Nikhil Panicker, Sethilkumar
Karuppagounder, Woonjoong Richard Kim, Minjee Kook, Hojae Lee, Gabsang
Lee, Catherine Foss, Subhash Kulkarni, Pankaj Pasricha, Martin Pomper
and Valina Dawson of the Johns Hopkins University School of Medicine;
and Chentian Shen of the Johns Hopkins University School of Medicine and
the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
This work was supported by the National Institute of Neurological
Disorders and Stroke (NS38377, NS082205 and NS098006) and the Morris K.
Udall Parkinson’s Disease Research Center.
Contact Rachel Butch (rbutch1@jhmi.edu) for a copy of the manuscript and interviews with the researchers
N:B- Content may have change in shape and size for publication