Vaccines and its usage to prevent disease: A relevant discussion

Dr. Shuvomoy Banerjee, Neucrad Health Desk July 17, 2020
“Prevention is better than cure” keeping this proverb in mind, doctors and scientists have been using vaccines for the last many years to protect millions of people around the world from several infectious disease. Currently, development of new and effective vaccines has gained immense importance particularly because of novel coronavirus infections.
Since, we are seeing vaccine related articles almost daily for the same and relevant reasons, we must know some of the vital questions regarding these vaccines first viz. 1) What is ‘Vaccine’? 2) When did it originate and what are its earlier known applications? 3) What are the various types of vaccines 4) How a vaccine helps our body’s immune system to fight against a disease? 5) What are the steps before a vaccine becomes available to people.
Let’s know more about these here:
What is a Vaccine?
A vaccine is a method of scientifically inactivating a living organism (microorganism) or its derivatives that are responsible for a disease, entering of which in the body helps to develop immunity against that particular disease. The method of administrating the vaccine is called ‘vaccination’.
The vaccine can be injected or sprayed into the nostrils or can be used as an oral solution.
Origin and Application of Vaccine:
Interestingly, the first attempt to make a vaccine began in India in the seventh century! Buddhist monks in India used to take very small amounts of snake venom to build immunity against snake bites. A special term, called “variolation” has been found in the history of ancient medicine. About two thousand years ago, in Central Asia, the dried pustules from smallpox patients were collected for the future preventive purpose of this disease. Later the pustule powder was applied on healthy people, who were found not to develop smallpox! This method was named as-“variolation”, derived from the Latin name for ‘varicella’ virus (the pathogen that causes smallpox). This ancient medical practice later spread to China, western Turkey, Africa and Europe.
The year 1897 became particularly significant in the history of medicine when an English medical scientist named Edward Jenner invented the smallpox vaccine scientifically. He applied the cowpox pathogen to the body of a healthy person. Since, cowpox has structurally similar entity with smallpox and surprisingly found that the person’s body developed immunity against smallpox.
In the 19th century, French scientist Louis Pasteur extended the research on vaccination methods in order to prevent other severe diseases. Importantly, in his laboratory he proved that it was possible to weaken and inactivate the pathogen by reducing its ability to infect. He applied this technique of using inactivated pathogens and successfully invented vaccines for chicken cholera, anthrax and rabies for the betterment of mankind.
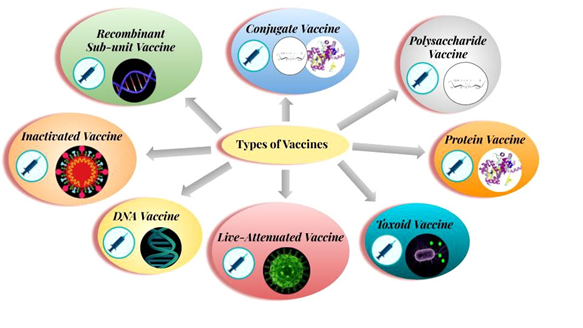
Different types of Vaccines:
The vaccines that are being used in various immunizations are:
- Live Attenuated Vaccine: A case where the pathogen is scientifically weakened and its pathogenic capacity is destroyed. When such pathogen enters human body, it helps to build immunity against that particular disease. This kind of vaccine is used to prevent measles, mumps and chickenpox. However, the pathogens used in this vaccine rarely regain their ability for infection and may cause the disease again.
- Inactivated Vaccine: Inactivating pathogens by heat inactivation, radiation or chemical process lead to such type of vaccines. Scientists use such vaccines to prevent diseases like Polio, Influenza, Hepatitis-A and Rabies.
- Sub unit Vaccine: It was possible to prevent plague with sub unit vaccine. In this type of vaccine, a part (any antigen) and not the whole complete pathogen is used as a vaccine.
- Toxoid Vaccine: Many bacteria release toxic chemicals, which harms the body. These chemicals are called ‘toxins’. Toxins can be scientifically ‘detoxified’ with the help of formalin and can be used as a vaccine. Toxoid vaccine for tetanus is known in usage.
- DNA Vaccine: Research on DNA vaccines is underway around the world, where the genetic material or DNA/RNA of the pathogen is used as a vaccine. There has been use of DNA vaccine against influenza.
- Recombinant Vaccine: This is somewhat similar to the DNA vaccine. However, the recombinant DNA vaccine is made with Recombinant DNA Technology (RDT) by adding the DNA sequence of the antigen of the required pathogen to the DNA of the virus or bacteria. The DNA of a virus or bacteria acts as a vector for cloning purpose. The diphtheria-pertussis-tetanus or DPT vaccine is a type of recombinant vaccine.
- Polysaccharide Vaccine: The vaccine is made from a long polysaccharide chain located in a bacterial capsule. This is a special type of inactivated sub-unit vaccine. There is use of polysaccharide vaccine to prevent diseases like pneumonia, meningitis etc.
- Protein Vaccine: Such vaccine is prepared by purifying essential proteins from viruses or bacteria or to make recombinant proteins. Hepatitis B disease is prevented with a protein vaccine.
- Conjugate Vaccine: A vaccine which is made with both polysaccharides and proteins of the bacterial cell wall. An example for this category is Haemophilus influenzae type-B vaccine.
How does this vaccine work?
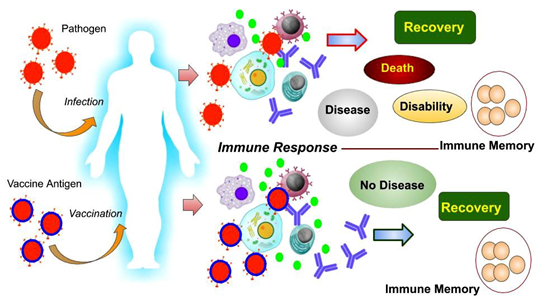
In general, pathogenic infections become crucial for the activation of our body’s immune system. Immune cells in our blood (such as neutrophils, eosinophils, macrophages, natural killer cells, B-lymphocytes, T-lymphocytes) are constantly fighting against pathogens. Macrophages swallow pathogens; B-lymphocytes produce antibodies to neutralize the infection, T-lymphocytes attack and destroy pathogens or infected cells. When the infection is under control, most of the pathogens die from the effects of such active immunity. The body heals and meanwhile some ‘memory cells’ are formed to prevent subsequent re-infection. But if the severity of the infection is high, the immunity gets defeated and may lead to the fatal consequences including death of the infected person.
Vaccines have lead to the solution of this problem! During vaccination, inactivated pathogens or its antigens are administrated in the body. Consequently, antibodies are made against that specific pathogen, immunity is activated, and even ‘memory cells’ are formed but no symptoms of disease are manifested. In short, our body stays healthy and develops an active immune system against those pathogens. Scientific studies have shown that once a vaccine is given, its effects may not last for a lifetime. Inactivated and live attenuated vaccines tend to lose effect over time. It has never been seen that a single vaccine does not produce enough immunity. Some vaccine doses are given every few years. This is called ‘booster dose’. Again, the influenza vaccine has to be taken every year. Because the character of the influenza virus changes rapidly, older vaccines do not work.
Vaccines for the prevention of Epidemics and Pandemic:
In context of Epidemiology, the spread of infection can be of two types:
A) When an infectious disease spreads to a specific geographical area, it is called an ‘Epidemic’
B) If the disease continues to spread almost all over the world, it is called ‘pandemic’. E.g. the current novel coronavirus infection!
Vaccination is very effective for preventing these two types of infection spread and is therefore an essential part of the public health system. In 1918, influenza pandemic killed nearly 4 million people worldwide. Influenza pandemic again re-emerged in 1958, 1967 and 2009. However, the death rate was much lower. Some scientists believe that the discovery and use of vaccines in that situation actually helped to reduce infection and deaths. Severe acute respiratory syndrome or SARS pandemic started in Asia in 2003 and spread to Europe and America. Again, the use of vaccines and antiviral drugs greatly reduced the infection rate. But now, again the Covid-19 Pandemic has become a threat to mankind and the daily death rate of people is escalating all over the world. Apart from regular monitoring of the infection, social distancing, quarantines and other treatment strategies, effective and safe vaccines are still awaited to prevent this pandemic.

Vaccine Preparation Methods and Clinical Trials:
Appropriate, safe and effective vaccines require a ‘long-term approach’. Following number of steps are involved in the development of a vaccine for a specific disease:
1. Exploratory stage: Scientists from the government or non-government organizations, universities, and pharmaceutical companies identify the pathogenic bacteria, viruses, or cancer cells and extract the necessary antigens from them to make vaccines.
2. Pre-clinical stage: Activation of immune response is monitored first by identifying the antigen of the pathogen and applying it over the cells via in-vitro tissue cultures. If good results are obtained, the next step is performed by conducting in-vivo or animal model (e.g. rats and monkeys) research to check the efficacy of vaccine.
* Necessary approval from the Ministry of Health and Indian Council of Medical Research or ICMR.
3. Phase I vaccine trial: At this stage, the vaccine is tested on small group (20-60 adults). The effect of the vaccine on infected people is also observed with necessary permission. If the vaccine is found to be effective and safe, then it reaches the next trial.
4. Phase II vaccine trial: The effects of the vaccine are checked in hundreds of people. Accompanying information such as the patient’s age, other illnesses, effects of other drugs during the trial and safety of the vaccine are thoroughly reviewed.
5. Phase III vaccine trial: The efficacy of the vaccine is checked in 1000-10,000 thousand people. At this stage, the rate of immune boost up by the vaccine and ability to make antibodies are examined carefully.
* Another round of government approval process
6. Phase IV vaccine trial: Pharmaceutical companies may run clinical trials once again if they wish even after receiving the approval.
7. Quality control: This step verifies the quality of the final stage of the vaccine.
8. Marketing: The vaccine is now released and available to the public.
References:
- Poland GA, Murray D, Bonilla-Guerrero R. New vaccine development [published correction appears in BMJ 2002 Jul 27;325(7357):195]. BMJ. 2002;324(7349):1315-1319. doi:10.1136/bmj.324.7349.1315
- Garmory HS, Perkins SD, Phillpotts RJ, Titball RW. DNA vaccines for biodefence. Adv Drug Deliv Rev. 2005;57(9):1343-1361. doi:10.1016/j.addr.2005.01.013
- Lee JS, Hadjipanayis AG, Parker MD. Adv Drug Deliv Rev. 2005 Jun 17; 57(9):1293-314. Epub 2005 Apr 15.
- Oyston P, Robinson K. The current challenges for vaccine development. J Med Microbiol. 2012;61(Pt 7):889-894. doi:10.1099/jmm.0.039180-0
- Kaufmann SH, McElrath MJ, Lewis DJ, Del Giudice G. Challenges and responses in human vaccine development. Curr Opin Immunol. 2014;28:18-26. doi:10.1016/j.coi.2014.01.009
- https://www.cdc.gov/vaccines/basics/test-approve.html