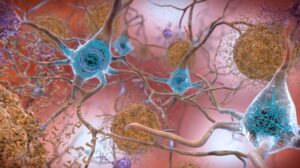
The First ever FDA approved CAR-T cell therapy for the treatment of Mantle Cell Lymphoma (MCL)

square grunge black fda approved stamp
Dr. Shuvomoy Banerjee, PhD. from Neucrad Health Desk; July 31,2020
‘TecartusTM’ has recently become the first ever approved CAR-T cell therapy by the US Food and Drug Administration (FDA). ‘TecartusTM’ therapy has been developed by Kite Pharma specifically for the treatment of relapsed or refractory Mantle Cell Lymphoma (MCL). The conventional name of this particular therapy is ‘brexucabtagene autoleucel’.
Before we know in detail about the clinical trial results and efficiency of this approved therapy, we must understand the basics of MCL and CAR-T cell therapy in general.
Mantle cell lymphoma:
In the human body, B-lymphocytes are a special type of leukocyte, which makes antibodies and maintains immunity against foreign pathogens. Several reasons including, chromosomal aberrations, gene mutations, or abnormal activity of the cell-cycle protein cycline D1 cause the amount of B-lymphocytes in the blood and lymph nodes to increase uncontrollably leading to lymph node cancer. This type of cancer is called “Non-Hodgkin’s Lymphoma”. Mantle cell lymphoma is just one type of this non-Hodgkin’s lymphoma. Such cancer spreads rapidly from one lymph node to another through metastasis. Without proper cancer therapy, the patient dies within a very short span of time.
Conventional ways of treating MCL:
- Chemotherapy:Different cancer drugs are treated individually or in combinations.
- Targeted therapy: Here, the functions of specific cancer cell proteins are prevented by using various protein inhibitors and the cancer cells are targeted by reducing their cell division capacity.
- Stem cell transplantation: Stem cell transplantation can be done in two ways-
- Autologous transplantation– In this case, new stem cells are collected from the patient’s bone marrow after high dose chemotherapy and re-introduced into the patient’s body. This technique destroys the cancer cells and disseminates the healthy cells in the body.
- Allogeneic transplantation– Stem cells are collected from donors after HLA (Human Leukocyte Antigen) match and transplanted into cancer patients.
- Immunotherapy:“CAR-T cell therapy” or ‘Adoptive T-cell therapy’ falls into this particular category. Here the cancer cells are destroyed using body’s immune system.
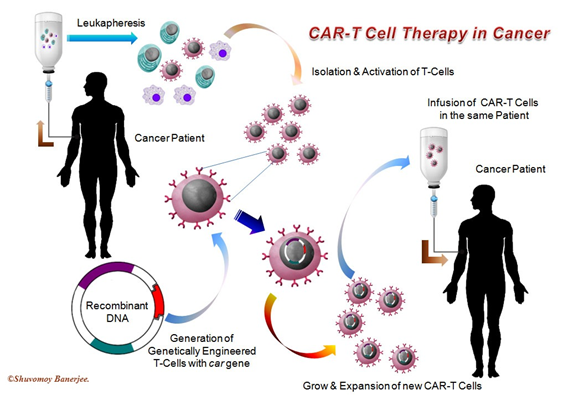
What is CAR-T cell therapy?
Immunity aids in attacking and destroying pathogens or germs which take entry in our body. Many important cells and cellular proteins are the key players for our body immunity and one of them is T-lymphocyte which targets foreign cells or antigens and destroy them by cell-mediated cytotoxicity. Of note, T-lymphocytes are specifically being used in Chimeric Antigen Receptor (CAR) T-cell therapy. It can be considered as a part of ‘cell therapy’ or ‘gene therapy’.
In this therapy, T-lymphocytes are first collected from the cancer patient’s body by “Leukopheresis” method. These collected T-lymphocytes are converted into CAR T-lymphocytes with the help of ‘Genetic Engineering’ in the laboratory. We know that CD-19 protein is found in the cell membrane of most cancer cells. Therefore, when the receptors for the CD-19 protein are added to T-lymphocytes by recombinant DNA technology (RDT), the tagged T-cells gain the ability to easily identify the cancer cells with CD-19 marker. These ‘Genetically Engineered’ T-lymphocytes are re-introduced into the patient’s body as an infusion in case of CAR-T cell therapy, where CAR-T lymphocyte identifies CD-19 protein of the patient’s cancer cells and destroy them.
Details about TecartusTM, its efficiency and side-effects in MCL therapy:
Kite Pharma undertook a project called ZUMA-2 for CAR-T cell therapy research and clinical trials. TecartusTM is the same technique used in adoptive T-cell therapy. Dr. Michael Wang, lead investigator of the Zuma-2 project and Professor of ‘Lymphoma and Myeloma department’ at the University of Texas MD Anderson Cancer Center, informed that despite the discovery of many advanced cancer drugs, there is still a lack of significant treatment for Mantle Cell Lymphoma. TecartusTM CAR T-cell therapy is quite effective in patients who have been suffering from this cancer for a long time or have recurrent cancer symptoms, and are not getting good response by the traditional treatment methods.
It was observed that 87% of total patients in the trial have responded very well with only single infusion dose of TecartusTM and remarkably 62% of the patients fully recovered after the therapy!
To check if TecartusTM therapy has any adverse effects on the human body, further necessary tests were performed. Interestingly, there is considerable side effects were found with this cell therapy. When CAR-T cells enter into the body divide rapidly, they produce large amounts of cytokines. This results in cytokine release syndrome (CRS). Simultaneously neurological toxicity also develops, leading to symptoms such as encephalopathy, hypertension etc. In trial, it was found that 18% of the participants had grade-3 CRS and 37% showed neurologic toxicity. However, despite these adverse side effects, TecartusTM therapy is considered to be an impeccable and effective ways of treating MCL currently. FDA has brought this therapy under the Risk Evaluation and Mitigation Strategy (REMS). This means that due to the considerable side effects of this therapy, the patient will have to take this therapy under the supervision of a specially trained oncologist on REMS program.
Apart from the above trials on Mantle Cell Lymphoma, phase-I / II clinical trials using TecartusTM have began for Acute Lymphoblastic Leukemia (ALL) and Chronic Lymphocytic Leukemia (CLL). Scientists, Researchers and Cancer patients are eagerly waiting for the final results of those trials.
References:
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-relapsed-or-refractory-mcl?utm_campaign=072420_PR_First%20Cell-Based%20Gene%20Therapy%20Approval%20For%20Relapsed%20or%20Refractory%20MCL&utm_medium=email&utm_source=Eloqua
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331-1342. doi:10.1056/NEJMoa1914347
- Miliotou AN, Papadopoulou LC. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol. 2018;19(1):5-18. doi:10.2174/1389201019666180418095526
- Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:2040620719841581. Published 2019 Apr 15. doi:10.1177/2040620719841581
- http://investors.gilead.com/news-releases/news-release-details/us-fda-approves-kites-tecartustm-first-and-only-car-t-treatment
- Bankhead C. CAR T-Cell Therapy Highly Active in Various Lymphomas. Am Health Drug Benefits. 2016;9(Spec Issue):19.
- https://www.yescartatecartusrems.com/