Oral Polio Vaccine (OPV) and the Inactivated Injectable Polio Vaccine (IPV) – a Briefing Note
By Banibrata Mukhopadhyay, PhD November 30, 2019
Poliomyelitis (Polio) usually affects the children under age 5 years without vaccination. The polio viruses cause paralysis or even death and are of 3 different types Polio virus: PV1, PV2 and PV3. The viruses generally enter through the mouth with eatables and drinks, water etc, multiply in intestine and passes through faeces.
The symptoms of polio are fever, fatigue, headache, vomiting, stiffness in the neck, pain in the limbs, and weakness in the limbs. If a child under 15 years of age suddenly shows signs of a floppy or weak arm or leg, immediate medical intervention is needed. There is no cure for polio.
There are two vaccines for polio: the Oral Polio Vaccine (OPV) and the inactivated inject able Polio Vaccine (IPV). IPV protect against all three types of polio viruses (types 1, 2 and 3) and hence is trivalent. Bivalent OPV (bOPV) targets type 1 and type 3, but not type 2. The previous old OPV (wild type) was used for immunization against only PV2 and hence termed as monovalent.
WHO Recommendations:
In May 2012, the World Health Assembly of WHO declared poliovirus eradication to be a programmatic emergency for global public health. WHO recommend that the use of OPV must eventually be stopped worldwide, starting with OPV containing type 2 PV (OPV type 2). At least one dose of IPV must be introduced, given in addition to OPV, to protect against type 2 PV and to boost population immunity against PV1 and 3.
The risk of paralytic disease due to OPV type 2 now outweighs its benefits.
One type of polio caused by OPV is called Vaccine Associated Paralytic Poliomyelitis (VAPP). For every birth cohort of 1 million children in OPV-only using countries, there are 2-4 cases of VAPP. This translates to an estimated 250-500 VAPP cases globally per year.
Another form of vaccine associated polio is the Circulating Vaccine Derived Poliovirus (cVDPV). These are mutated versions of OPV which can cause paralysis and spread from person-to-person. The wild type monovalent OPV is thus eradicated globally by WHO on 1999. Bivalent OPV will continue to target the remaining polio types (types 1 and 3). The switch from trivalent OPV to bivalent OPV will significantly reduce the risk of VAPP and cVDPV.
IPV is recommended in addition to the oral bOPV. IPV does not replace the oral vaccine.
The proposed schedule of bOPV and IPV as recommended by WHO for the infants even for those born prematurely:
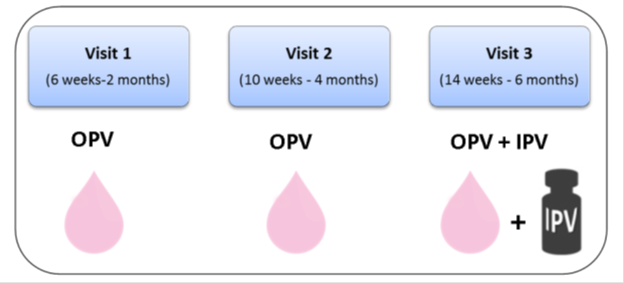
The global effort to introduce IPV in all countries has been complicated by major shortfalls in the production of IPV as well as demand for IPV for use in mass campaign supplemental immunization activities (SIAs). A global shortage of IPV has resulted in introduction delays in approximately 20 countries worldwide until 2017. At least 29 other countries are expected to experience national stock outs of IPV before being resupplied.
References:
MMWR Morb Mortal Wkly Rep 2016; 65:934–38.
The Journal of Infectious Diseases, Volume 216, Issue suppl_1, 1 July 2017, Pages S86–S93, https://doi.org/10.1093/infdis/jix133