Fabiflu: India’s First Approved Oral Antiviral Drug to combat Covid-19 pandemic

Neucrad Health June 23, 2020
The latest addition to the coronavirus treatment strategy is the launch of an oral antiviral drug Flavipiravir (Flavipiravir)! At a time when the number of Covid-19 patients in India has exceeded 4 lakhs and the mortality rates for the same is on the rise, Glenmark Pharmaceuticals (a well-known Indian Biopharma company), is going to bring this drug to the market. After a lot of research works, this Mumbai-based pharmaceutical company has received the necessary clearance from the Drug Controller General of India (DCGI, India), for the use of Flavipiravir in Covid-19 therapy. The brand name of the drug is going to be ‘Flaviflu‘.
Fabiflu: About the Drug, its Past and Chemical Composition
Heterocyclic aromatic compounds called Purine and Pyrimidine are found in our body. These act as nitrogen bases in the structure of cellular DNA and RNA. Interestingly, the chemical composition of Fabiflu compound is considered as ‘analogue’ as the drug is very close to the structure of Purine base. This broad spectrum antiviral, Flavipiravir (Fabiflu) is not a newly discovered drug. Already, there was a wide use of this drug in Bangladesh and United Arab Emirates (UAE). The drug is now awaiting clearance in Egypt and Jordan. Notably, Japan approved Flavipiravir for influenza treatment in 2014. Moreover, in current Covid pandemic situation, Japan has once again used Flavipirevir but on a “compassionate use” basis where a drug can be used in an extreme case with special restrictions and without approval. They reported 88% success rate for their clinical trials on around 7,050 Covid-19 patients which are quite remarkable indeed!
With the initiative of the Indian Ministry of Health and the Indian Council of Medical Research (ICMR), Glenmark Pharmaceuticals began their research on Flavipiravir and its experimental application, as a solidarity trial in the novel coronavirus infection. According to them, Flavipiravir has done extremely well in their clinical trials. The company claims that this drug can be used on Covid-19 patients with mild to moderate symptoms. Interestingly, their studies have shown that the drug helps to reduce the incidence of Covid-19 disease in patients aged 80-90 years alongside young patients. In addition, due to the patient’s co-morbidity, the use of flavipiravir is particularly effective where coronavirus infections are a high risk factor. Studies have also depicted that flavipiravir generally helps to reduce the amount of virus growing in the patient’s body within 4 days of drug application.
Mr. Glenn Saldanha, Chairman and Managing Director of Glenmark Pharmaceuticals, is quite optimistic about the outcome of the recovery trial of Flavipiravir. According to him, speculating the current scenario, the way corona infection is spreading throughout India, the drug will be a great deal in preventing the infection!

How will Fabiflu prevent novel coronavirus infection?
Novel Coronavirus mainly infects lung cells. Once the virus enters the cell, it begins replication of its genetic material (RNA molecule) by collecting purine nucleosides from the cell. We have already discussed that Fabiflu is structurally similar to purine. In this case, a competition between the ‘purine nucleoside’ and the ‘purine analog’ begins while replication because of the same structural appearances. The RdRp (RNA-dependent-RNA-Polymarese) enzyme of the virus is interrupted by the addition of Fabiflu instead of ‘purine nucleoside’. Notably, Fabiflu is not active outside the cell. This inactive state is called ‘pro-drug’. After entering the cell, this ‘pro-drug’ is transformed into an active drug by undergoing the necessary biochemical reactions. This Active Fabiflu actually helps to prevent the viral infection.
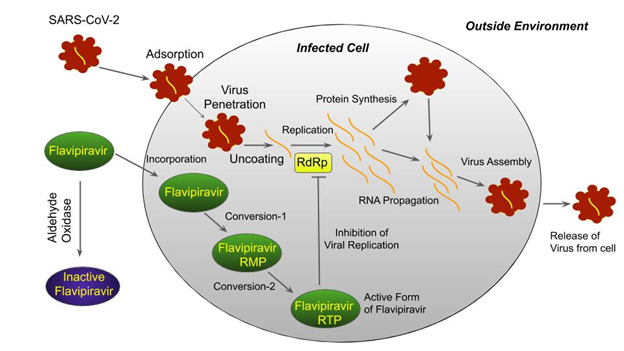
Probable Mechanism of Fabiflu action inside coronavirus Infected cells: Fabiflu is inactivated by the effect of aldehyde oxidase enzyme outside the infected cell. After entering the cell, it undergoes various biochemical reactions to become active ‘flavipiravir-RTP’. This active compound inhibits the action of the RdRp (RNA-dependent-RNA-Polymarese) enzyme. As a result, replication of coronavirus is stopped.
Usage of Fabiflu:
The drug Fabiflu can be used as an oral tablet in the treatment of corona. Intravenous injection (IV) will not be required. Its price has been kept affordable for the Indian public as each tablet will cost 103 INR only. However, this drug can only be used with the permission of a physician. The recommended dose of the drug is 1800 mg (can be used twice a day) and then 800 mg for two weeks after judging the symptoms by the Physician. The Glenmark Company has added that they are conducting another clinical trial of “combination therapy” with Fabiflu with other antiviral called “Umifenovir”. It remains to be eagerly seen what effect this therapy will have on Covid-19 patients. Scientists believe that if coronavirus replication can be stopped at an early stage with Fabiflu, it will be possible to avoid the next terrible advanced stage of corona infection i.e. ‘cytokine storm’. In conclusion, all eyes are on Fabiflu and its effectiveness as a magic pill to combat Covid-19 in the upcoming weeks.

References
- https://www.glenmarkpharma.com/sites/default/files/Glenmark-FabiFlu-Press-Brief.pdf
- https://www.indiatvnews.com/business/news-fabiflu-tablets-dosage-covid-19-treatment-favipiravir-glenmark-indian-company-gets-approval-628184
- https://www.livemint.com/market/mark-to-market/glenmark-s-fabiflu-is-first-in-the-market-but-growth-opportunity-limited-11592810808435.html
- https://www.indiatoday.in/india/story/coronavirus-treatment-drug-glenmark-fabiflu-favipiravir-launch-india-rs-103-per-tablet-reduce-viral-load-1691066-2020-06-20
- https://indianexpress.com/article/india/glenmark-to-market-the-antiviral-under-the-brand-name-fabiflu-6470539-6470539/
- Du YX, Chen XP. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection [published online ahead of print, 2020 Apr 4]. Clin Pharmacol Ther. 2020;10.1002/cpt.1844. doi:10.1002/cpt.1844
Author Profile:
Dr. Shuvomoy Banerjee, PhD
Dr. Shuvomoy Banerjee has taught as an Assistant Professor in the Department of Virology and Immunology at Amity University, Delhi. He holds a PhD degree in Cancer Biology from Jadavpur University and later did Postdoctoral Research in Viral Oncology from University of Pennsylvania School of Medicine as well as from City of Hope National Cancer Institute (California). Currently, the author is actively involved in teaching, scientific writing and cancer research awareness programs.