Editas’s CRISPR Study on Human Follows Norm
November 5, 2018
Last week, a Chinese doctor claimed that he edited first time the genomes of twin girls using CRISPR technology. But he didn’t follow any regulatory norms for his study in human. Research without ethics is very dangerous towards mankind.
But Editas Medicine, headquartered in Cambridge, MA is following a different process for bringing a clinical trial using CRISPR technology (CRISPR/Cas9 and CRISPR/Cpf1). U.S. Food and Drug Administration (FDA) accepted Editas’s Investigational New Drug (IND) application. Under regulatory norms, Editas, co-developing with Allergan, is going to study EDIT-101, a CRISPR-based therapy, on human with a rare genetic disorder named Leber congenital amaurosis 10 (LCA10) that leads to blindness. It is estimated that less than three out of 100,000 children have this disease at birth. LCA10 is an inherited retinal degenerative disease characterized by severe loss of vision at birth. Other abnormalities, including roving eye movements and sensitivity to bright light, are also evident with this disease. LCA10 is caused by a mutation in the CEP290 gene.
CRISPR technology can be used to edit genes within cell. Editas is aiming to delete the mutation in the CEP290 gene using CRISPR within cells. It is assumed that it will be able to restore the normal protein expression and function of the photoreceptor cells. By this technique it may be able to arrest any further loss of vision.
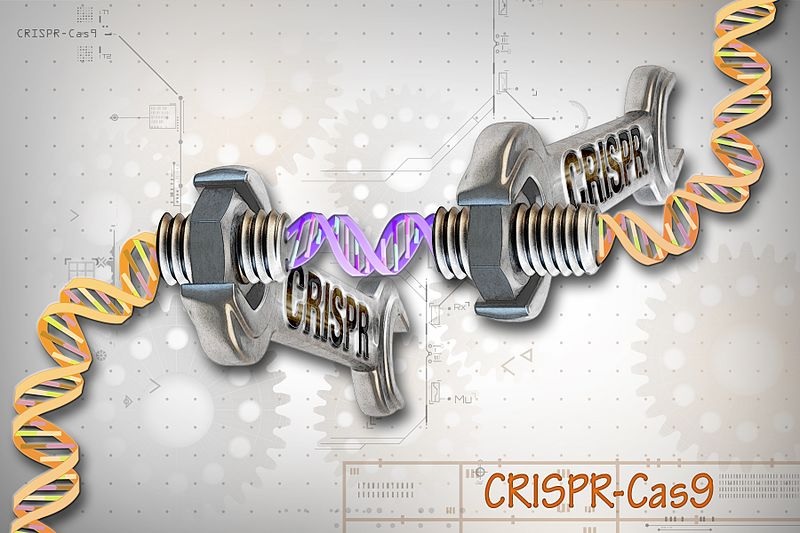
About Image : It is for a model representation purpose only. CRISPR-Cas9 is a customizable tool that lets scientists cut and insert small pieces of DNA at precise areas along a DNA strand. The tool is composed of two basic parts: the Cas9 protein, which acts like the wrench, and the specific RNA guides, CRISPRs, which act as the set of different socket heads. These guides direct the Cas9 protein to the correct gene, or area on the DNA strand, that controls a particular trait. This lets scientists study our genes in a specific, targeted way and in real-time.
Image Credit: Ernesto del Aguila III, NHGRI. CC-BY-2.0