COVID-19: The unsolved chapter of science

Sourav Mukherjee April 8, 2020
Coronaviruses (CoVs) create various types of diseases like respiratory, enteric, renal, and neurological disorders by affecting humans as well as animals[1]. Severe acute respiratory syndrome-related coronavirus (SARS-CoV) initially appeared in people in 2002-2003, affected above 8000 people, and resulting in approximately 800 deaths[1, 2]. Middle East respiratory syndrome coronavirus (MERS-CoV), a genealogy C beta-CoV, was causing a severe respiratory syndrome in 2012[3]. A novel coronavirus, called SARS-CoV-2 (COVID-19), was detected in sufferers with severe pneumonia in Wuhan, China, in December 2019[4]. On 11th March 2020, WHO declared that COVID-19 could be identified as a pandemic and also mentioned that this is the first pandemic caused by a coronavirus( Source: WHO). According to WHO, the total number of confirmed cases of COVID-19 is 1,056,159 and resulting in 57,206 deaths in entire 208 Countries, areas, or territories with cases (Updated: 4 April 2020, 23:48 GMT+5:30). Few essential characteristics of COVID-19 are mentioned below-
- All coronaviruses encode a spike glycoprotein (S), which attaches to the host-cell receptor, followed by mediating membrane fusion as well as viral incorporation and resulting in the antibody neutralization in our body[5].
- Trimeric S protein is consists of monomers of nearly 180 kDa and S1, S2 are two subunits of it which helps in mediating addition and membrane fusion, sequentially. N-terminal domain (NTD) and C-terminal domain (C-domain) are the two self-governing regions of N- and C- concluding parts of S1 fold. Both NTD and C-domain has the potential to act as the receptor-binding domain (RBD), although the virus itself controls the preference[1]. Both SARS-CoV and MERS-CoV, bind to their receptors with the help of C-domain[6, 7].
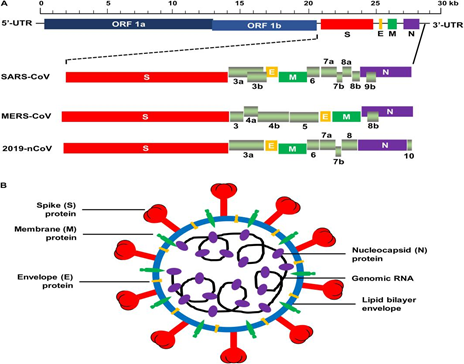
Fig. 1: Schematic arrangement of SARS-CoV, MERS-CoV, and 2019-nCoV[8]
- Recently, Hoffmann et al. described that hACE2 (Human ACE2) is the entrance receptor of SARS-CoV-2, and also TMPRSS2, a serine protease, is essential for SARS-CoV-2 S activation[9].
Origin of COVID-19:
A group of researchers examined every important SARS-CoV-2 characteristics like the optimized RBD and polybasic splitting section, in relevant CoVs in nature. After analyzing the results, the researchers did not consider SARS-CoV-2 as a laboratory-based product[10]. Hence, The novel SARS-CoV-2 which appeared in China last year is the outcome of natural evolution.
Protective measures against SARS-CoV-2:
According to WHO, to protect you and your family, everyone should follow the instructions given below ( Source: WHO)-
- Maintain social distancing
- Wash your hands frequently
- Avoid touching eyes, nose and mouth
- Practice respiratory hygiene
- If you have fever, cough and difficulty breathing, seek medical care early
- Stay informed and follow advice given by your healthcare provider

Recent investigation on finding out a possible cure for COVID-19:
Researchers all over the world are trying to develop a potential treatment for COVID-19. A possible treatment can be of various types, including potential drug discovery (both novel and existing), vaccine development, immunotherapy, etc. Here are the few recent updates ( up to 06th April 2020) on the COVID-19 research-
Drug Doscovery (both novel and existing):
- Scientists have discovered that Ivermectin is an inhibitor of SARS-CoV-2, and it has the potential to influence a ∼5000-fold decrease in viral RNA at 48 hours by applying once to Vero-hSLAM cells 2 hours post-infection with SARS-CoV-2 [11]. Ivermectin is an FDA-approved anti-parasitic agent and has broad-spectrum antiviral activity in vitro. Therefore, it can be beneficial for humans and require additional investigation.
- Remdesivir and Chloroquine, two promising antiviral drugs, are profoundly useful in handling SARS-CoV-2 infection in vitro, recent research revealed (EC90 value of Remdesivir and Chloroquine against SARS-CoV-2 in Vero E6 cells was 1.76 μM and 6.90 μM respectively) [12]
- Recent investigation disclosed that Hydroxychloroquine medication is significantly linked with the reduction in the viral carriage in SARS-CoV-2 sufferers, and Azithromycin strengthens its effect [13]. Another research also demonstrated that Hydroxychloroquine could inhibit SARS-CoV-2 in vitro more effectively than Chloroquine [14]. Few safety concerns regarding the usage of Hydroxychloroquine and Chloroquine are risk of cardiac arrhythmias, retinal damage, especially with long term use, caution in patients with G6PD deficiency, diabetics, etc [15].
Apart from this, several drugs are on the process of clinical trials including-
| Condition or disease | Intervention/treatment | Phase | ClinicalTrials.gov Identifier |
| COVID-19 | Drug: Deferoxamine | Phase 1 Phase 2 | NCT04333550 |
| COVID-19 | Drug: Siltuximab Drug: Methylprednisolone | Phase 2 | NCT04329650 |
| COVID-19 | Other: hyperimmune plasma | Not Applicable | NCT04321421 |
| COVID-19 | Drug: DAS181 | Not Applicable | NCT04324489 |
| COVID-19 Pneumonia | Drug: Best supportive Care (BSC) + IFX-1 Drug: Best supportive care only | Phase 2 Phase 3 | NCT04333420 |
| CODID-19 | Drug: 1: Naproxen Drug: 2: Standard of care | Phase 3 | NCT04325633 |
| COVID-19 Pneumonia | Drug: Tocilizumab Injection | Phase 2 | NCT04317092 |
| COVID-19 | Drug: Sarilumab Drug: Placebo | Phase 2 Phase 3 | NCT04315298 |
| COVID-19 | Biological: NK cells,IL15-NK cells,NKG2D CAR-NK cells,ACE2 CAR-NK cells,NKG2D-ACE2 CAR-NK cells | Phase 1 Phase 2 | NCT04324996 |
These are the only few examples, and many more clinical trials are going on worldwide( Source: ClinicalTrials.gov).
Vaccine Development:
Few clinical trials intend to develop a universal vaccine for SARS-CoV-2 are-
| Condition or disease | Intervention/treatment | Phase | ClinicalTrials.gov Identifier |
| Treat and Prevent Covid-19 Infection | Biological: Pathogen-specific aAPC | Phase 1 | NCT04299724 |
| Pathogen Infection Covid-19 Infection | Biological: Injection and infusion of LV-SMENP-DC vaccine and antigen-specific CTLs | Phase 1 Phase 2 | NCT04276896 |
| COVID-19 | Drug: BCG Vaccine Drug: Placebo | Phase 3 | NCT04328441 |
| COVID-19 | Biological: Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) | Phase 1 | NCT04313127 |
| Corona Virus Infection Immunisations | Biological: mRNA-1273 | Phase 1 | NCT04283461 |
| Coronavirus | Biological: ChAdOx1 nCoV-19 Other: Saline Placebo | Phase 1 Phase 2 | NCT04324606 |
Research and Development in India
In India, scientists are also working hard to find out a cure for SARS-CoV-2 infection. In combating SARS-CoV-2, the contribution of Indian researchers are briefly described here-
- A low-cost paper-strip test, developed by the Institute of Genomics and Integrative Biology of Delhi, could identify COVID-19 within an hour [16]. It could achieve India’s accelerated requirement for testing as each test would cost ₹500 (US$7.00).
- Pune-based molecular diagnostic company Mylab Discovery Solutions got the RT-PCR tests’ validation from the National Institute of Virology and the Indian Council of Medical Research (ICMR) [17]. It became the first Indian company to get it. The test needs 2.5 hours and costs Rs 80,000 for a 100 test kit.
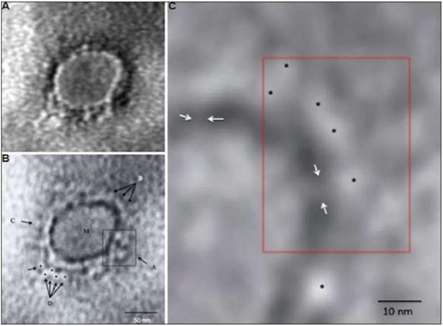
- India has published images of the coronavirus taken by the National Institute of Virology (NIV) investigators in Pune [18].
- Investigators have isolated 11 strains of the SARS-CoV-2 at the National Institute of Virology (NIV), Pune [19]. Therefore India has become the fifth country to do it successfully.
- To combat SARS-CoV-2, India’s Council for Scientific and Industrial Research (CSIR) has made a collaboration with multiple companies, like TCS, BHEL, and Cipla[20].
- Defence Research and Development Organisation (DRDO) has taken part in combating the SARS-CoV-2 by making high-security face masks to full body assurance suits and multi-patient ventilators [21].
- To fight COVID-19, ISRO has acknowledged by resting launches and turning supplies to generate ventilators and hand sanitizer [22]. Several IITs and IISc are also involved in making ventilators and PPEs, full-face shields, N95 masks, robotic units, drones, low-cost testing kits, handheld temperature measuring units, ICU beds, medical waste disposal for isolation wards, disinfection showers, mobile apps, etc [23, 24].
Author’s Note:
Please follow the official guideline carefully regarding COVID-19 and check the website regularly in case if there is any update. Stay home and stay safe.

References:
1. Ou, X., et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications, 2020. 11(1): p. 1-12.
2. Wang, M., et al., SARS-CoV infection in a restaurant from palm civet. Emerging infectious diseases, 2005. 11(12): p. 1860.
3. Zaki, A.M., et al., Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine, 2012. 367(19): p. 1814-1820.
4. Zhu, N., et al., China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med, 2020. 382(8): p. 727-733.
5. Li, F., Structure, function, and evolution of coronavirus spike proteins. Annual review of virology, 2016. 3: p. 237-261.
6. Li, F., et al., Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science, 2005. 309(5742): p. 1864-1868.
7. Lu, G., et al., Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature, 2013. 500(7461): p. 227-231.
8. Wang, N., et al., Subunit vaccines against emerging pathogenic human coronaviruses. Frontiers in Microbiology, 2020. 11: p. 298.
9. Hoffmann, M., et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 2020.
10. Andersen, K.G., et al., The proximal origin of SARS-CoV-2. Nature Medicine, 2020: p. 1-3.
11. Caly, L., Druce, J.D., Catton, M.G., Jans, D.A., Wagstaff, K.M., The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research, 2020.
12. Wang, M., et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research, 2020. 30(3): p. 269-271.
13. Gautret, P., et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents, 2020: p. 105949.
14. Yao, X., et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical Infectious Diseases, 2020.
15. Tim Smith, J.B., Tony Prosser, COVID-19 Drug Therapy. Clinical Drug Information | Clinical Solutions , Elsevier, 2020.
18. Prasad, Sharda, et al. “Transmission electron microscopy imaging of SARS-CoV-2.” The Indian journal of medical research (2020).
19. https://www.icmr.nic.in/sites/default/files/MediaReport_COVID19.pdf
22. https://www.space.com/india-space-agency-stops-launches-makes-ventilators-coronavirus.html
23. https://qz.com/india/1829905/the-iits-are-stepping-up-to-help-india-battle-coronavirus/