The European Commission gives Approval for Ebola Vaccine
By Neucradhealth News Desk November 17, 2019
Continuous research and painstaking efforts for more than two decades have finally brought positive results in the field of Ebola virus prevention. Yes, you have guessed it correct; The European Commission has finally approved the Ebola vaccine- Ervebo developed by Merck on 11th November 2019. Currently, research scholars and doctors in the Republic of the Congo are using the vaccine under a “compassionate use”, which is similar to clinical trials in the research protocol. It took less than a month after European Medicines Agency advocated Ervebo for licensing. Scientists and doctors all across the globe welcomed this move, as it would put to an end the extreme sufferings associated with the Ebola disease.
How did Merck react with the approval of Ervebo vaccine?
Ken Frazier, Chairman and chief executive officer of Merck, was extremely happy about the latest development. He opined that “The European Commission’s marketing authorisation of Ervebo is the result of an unprecedented collaboration for which the entire world should be proud.” He further added that this development of Ervebo vaccine is a historic milestone in the history of humankind.
Now, Merck is ready to co-ordinate with the Food and Drug Administration (FDA) in the United States, along with controlling agencies in different African countries for the licensing of this exceptional vaccine. They will also work with the World Health Organization (WHO) for the prequalification of Ervebo vaccine in different countries. This step is essential to prove that the vaccine is safe for use, and it would not lead to any severe complications or adverse health effects. The prequalification of vaccines acts as guidance for developing countries for the introduction of the injection in their nation.
Why is the development of Ebola Vaccine a priority for the European Commission?
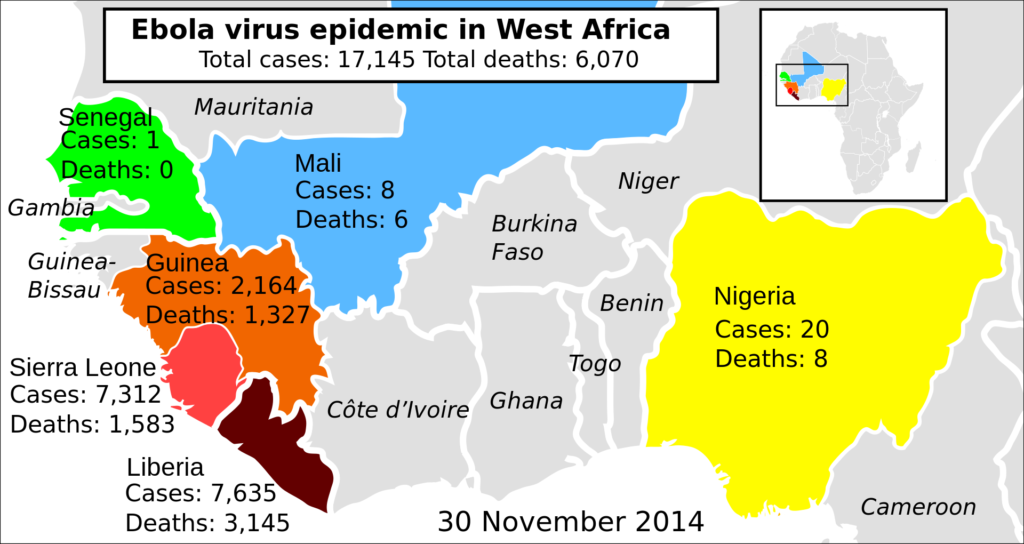
The licensing of Ervebo vaccine was a priority for the European Commission since the outbreak of this disease has created havoc in several African countries. Doctors first detected Ebola virus in Sudan and the Democratic Republic of Congo during 1976. During 2014-16, the deadly virus infected an estimated 28000 patients. More than 11000 of these victims succumbed to this disease. It was the biggest outbreak of Ebola since the documentation of this disease. The FDA now has time till March 2020 to decide the full-fledged introduction of the Ebola vaccine. However, health experts are suggesting that the governing body will issue its verdict much before the deadline.
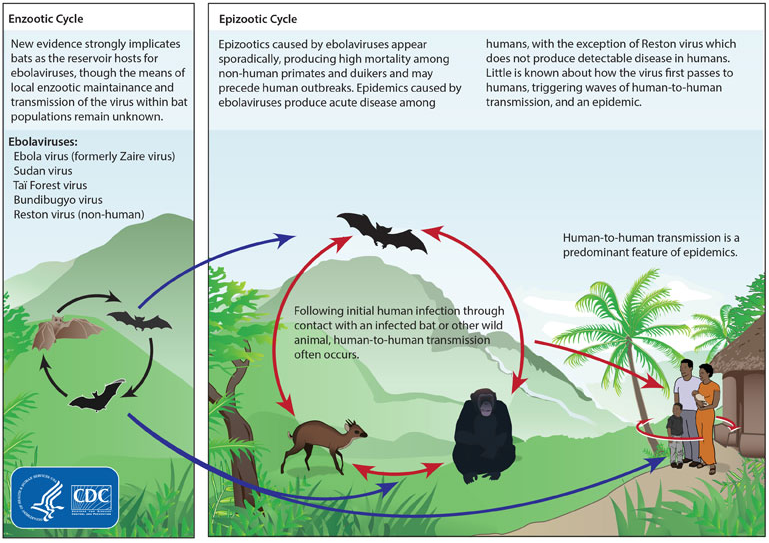
Basic information about Transmission of Ebola virus
Ebola is a deadly virus belonging to the Filoviridae family. It originated among African fruit bats, and later transmitted to human beings as well. Scientists also refer to it as Filovirus considering the family of the pathogen. There are six subtypes of Ebola virus- Bundibugyo, Reston, Sudan, Taï Forest, Zaire, and Bombadil virus. Out of these six subtypes, only four of them infect human beings- Ebola, Sudan, Taï Forest, and Bundibugyo. Reston virus causes infection in nonhuman primates like pigs, and Bombadil Ebola virus is the last-identified subtype present primarily in bats. It is a zoonotic infection, as it gets transmitted from animals to human beings. Chimpanzees, forest antelopes, porcupines, monkeys, and gorillas carry the virus to human beings. The animals’ caretakers can get infected to this disease while coming in contact with their blood and body fluids of infected animals.

What are the symptoms of Ebola infection?
The Centres for Disease Control and Prevention opined that the symptoms of Ebola infection usually manifest 8 to 10 days after exposure to the germs. Detailed below are the primary symptoms of Ebola infection.
- Diarrhoea
- Fever
- Headache
- Joint and muscle aches
- Raised rash
- Red eyes
- Chills
- Weakness
- Chest pain
- Sore throat
- Severe weight loss
- Internal bleeding
- Bruising
- Bleeding through eyes. As the disease becomes more complicated bleeding through ears, nose and rectum can also occur.
This was a brief about the Ervebo vaccine developed by Merck. Stay protected from this infection and lead a tension-free life.