Recent advancements in COVID-19 treatment regimes, research and development in global and Indian Scenario.

Pritam Mukherjee Neucrad Health, April 4, 2020
Corona Virus Disease 2019 or COVID-19 is a recent pandemic spread across 200 countries around the globe affecting more than 1 million people with more than 56000 deaths and 225000 recovered patients worldwide. This is a global emergency which is underpinned by the fact that not only the causative virus (SARS-CoV-2) is highly contagious but also there aren’t any unifying cures for COVID-19. Amidst these chaotic situations prevailing worldwide, scientist, researchers and doctors are working tirelessly to combat with the disease.
In this article we are going to discuss some recent advancements (till 31st March, 2020) in COVID -19 research and treatment regimes and scenario of Indian research and development.
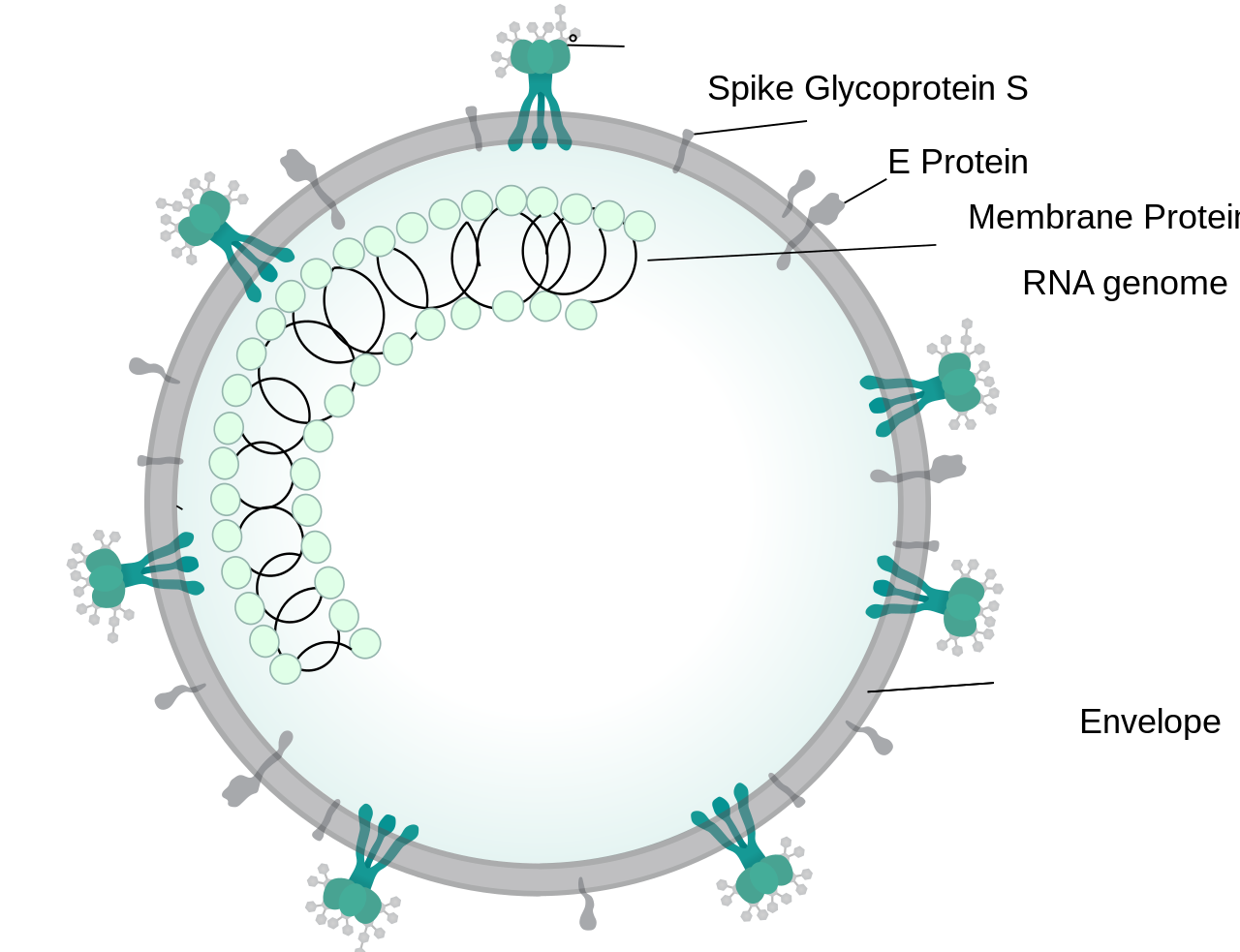
SARS-CoV-2 or 2019-nCoV, the causative virus of COVID-19 is an enveloped virus that has a glycoprotein spikes (which appears like crown when viewed under electron microscope, Latin: Corona – Crown) and bears a positive sense single stranded RNA genome which belongs to β-coronavirus subgroup (Order – Nidovirales, Family – Coronaviridae, Subfamily- Coronavirinae) [1]. The genome of SARS-CoV-2 is similar to other coronaviruses such as SARS-CoV and MERS-CoV which are responsible for Severe Acute Respiratory Syndrome (SARS, outbreak in 2003) and Middle East Respiratory Syndrome (MERS, outbreak in 2012) [2]. Like them SARS- CoV-2 encodes some structural proteins such as spike glycoproteins, and some non structural proteins, that includes 3-chymotrypsin like protease, papain like protease, helicase and RNA Dependent RNA Polymerase [3].
Treatment regimes
As of now coronavirus treatment is supportive. There aren’t any unique medicines available to combat with the disease. Researches over the years on SARS and MERS have started acting as primer for the COVID-19 treatment. Based on such researches following categories of treatment regimes are being taken into account and researches are on its peak. Here we list some active research and development pertaining to the major treatment regimes, which are as follows –
- Repurposing of drugs
- Immunotherapy and vaccinations.
- Monoclonal antibodies
- Convalescent Plasma
- Vaccines
Repurposing of existing Drugs:
The idea of repurposing antiviral drugs can be traced from the preliminary examinations of the genomic sequences of SARS-CoV-2. The results indicate that catalytic site of those 4 enzymes are highly conserved and share a significant level of sequence similarity with the corresponding SARS and MERS enzymes [4]. Spikes are indispensable for virus entry into the host cell and the non structural proteins are basically the key enzymes for viral life cycle [5]. These proteins has offered a potential drug targets for SARS and MERS and as SARS-CoV-2 is similar to those it is assumed that those can serve as drug targets for COVID-19 also [3]. It is of a special interest that whether the host cell angiotensin converting enzyme 2 (ACE 2) which act as receptor for virus entry can also be a potential target or not, researchers suggested that chronic use of ACE inhibitors and Angiotensin receptor blockers which is otherwise used for maintaining blood pressure may increase the severity of COVID-19 detected patients [6].
Below mentioned are some existing drugs and their targets that are being repurposed in some parts of the world or the clinical trials that has been initiated, for extensive list of candidates of repurposing existing drugs for COVID-19, interested readers can consult the supplementary information table 1 provided by Le and Clercq, Nature Reviews Drug Discovery 2020, 19: 149-150.
- Favipiravir (T-705) – It is a Guanosine analogue that inhibits viral RNA replication by targeting their RNA Dependent RNA Polymerase. Presently this drug’s been approved for Influenza, Ebola, yellow fever, Chikungunya, Norovirus and Enterovirus [4]. Researchers have used this drug to test its efficacy in vitro where they have found significant activity( EC50= 61.88μM in Vero E6 cells) [7]. That is why randomized clinical trial has started with patients with COVID-19 to test the efficacy of Favipiravir with Interferon α (ChiCTR2000029600) and Favipiravir with Baloxavir marboxil (ChiCTR2000029544).
- Remdesivir (GS-5734) –It is an Adenosine derivative and has a broad spectrum activities against RNA viruses such as SARS and MERS in cell culture and animal models and clinical trial have been performed for Ebola. Recent studies reported that Remdesivir inhibited SARS-CoV-2 significantly (EC50 = 0.77 μM in Vero E6 cells) [7]. Recovery of one COVID-19 affected US patient by intravenous injection of Remdesivir has been also reported [8]. Two phase 3 trials were also been initiated in patients affected by COVID-19 in early February to check the efficacy of intravenous Remdesivir which is likely to be completed in April 2020 (NCT04252664 and NCT04257656).
- Lopinavir-Ritonavir – These are HIV protease inhibitors and have shown significant activity in vitro and in animal model against SARS and MERS [9]. They are thought to bind to an important replication enzyme, M protease [10]. An open label, randomized, controlled clinical trials have been carried out with (n=199) COVID-19 affected patients. Where the researchers found out no significant difference from standard cared patients with Lopinavir-Ritonavir treated patients [11]. Moreover using HIV viral protease is debatable as HIV protease belongs to aspartic protease family but CoVs protease belongs to cysteine protease family [3].
- Chloroquine and Hydroxychloroquine – These are classified as antimalarial drug. These drugs individually have in vitro activity against SARS-CoV-2 and it is thought to inhibit viral enzymatic processes, viral protein glycosylation, virus assembly and virus release [9]. In one in vitro data it has been reported that hydroxychloroquine have higher antiviral activity compared to chloroquine [12]. In addition to that it has also been reported in one open- label, non-randomized clinical trial of measuring the efficacy of hydroxychloroquine alone and in combination with Azithromycin (a macrolide antibacterial) the data suggested improved recovery of patients treated combination of these two drugs. Azithromycin is thought to prevent bacterial superinfection [13]. Use of these drugs (choloroquine or hydroxycholoroquine) comes with a warning of cardiac arrhythmias, risk of retinal damage, caution in diabetics and G6PD deficiency. Recently US FDA has approved the use of choloroquine and hydroxycholoroquine for emergency COVID-19 treatment [9].
Immunotherapy and Vaccines

These regimes interplay with the host immune system. Three possibilities under this regime are treatment with monoclonal antibodies (such as Tocilizumab), treatment with COVID-19 Convalescent Plasma or prior vaccinations. Recent developments are:
Monoclonal Antibodies (mAbs)- Tocilizumab is an Interleukin 6 (IL 6) receptor blocking antibody [14]. Rationale of using this is underlined by the fact that IL 6 is a marker of inflammatory signature of the serum of severely affected COVID-19 patients with acute respiratory problem [15]. Society for Immunotherapy of Cancer (SITC) have encouraged the usage of this IL 6 receptor inhibitory mAbs like tocilizumab and other FDA approved IL 6 inhibitong mAbs which are otherwise used against various conditions such as rheumatic disease. Preliminary data from a study where 21 patients were treated with tocilizumab added in their standard COVID therapy suggest that this drug might have a clinical benefit as adjunctive therapy [16]. Risk includes GI perforation and hepatotoxicity [9]. Immunomodulating agents such as Interferon α, Sarilumab , etc are being evaluated as adjunctive therapy [17, 18].
COVID-19 Convalescent Plasma This is a type of passive antibody therapy. There has been a long history of using passive antibody therapy that dates back to 1890s and was the only way by which patients were treated before the development of antimicrobial therapy in the 1940s [19]. The possible mechanism of action of this therapy is by viral neutralization, and possibly antibody dependent cellular cytotoxicity and/or phagocytosis. The source of the anitibody for SARS-CoV-2 is human convalescent sera from those patients who have recovered from COVID-19 [20]. There is one report from China where 5 critically ill patients’ clinical conditions were improved when they were treated with convalescent plasma [21]. US FDA also approved emergency use of seriously ill patients with this therapy [22]. But large scale use of this therapy comes with ethical concerns.
Vaccines – There are presently no approved vaccines but researches developed some leads. As of 20th March 2020, the report published by WHO reported 2 candidate vaccines(one developed jointly by CanSino Biological Inc. and Beijing Institute of Biotechnology which is a non-replicating viral vector type vaccine and the other one is developed jointly by Moderna and NIAID which is a mRNA based vaccine) which are under clinical trial. WHO have also stated 42 candidate leads for vaccines and they are of different types such DNA based, inactivated, live attenuated, non replicating and replicating viral vector and protein subunit based and are under preclinical stage [23].
Indian research and development scenario in combating COVID–19
India is the 5th country who isolated the clinical isolates of SARS-CoV-2 at National Institute of virology labs [24]. Pune based company Mylab Discovery Solutions have developed SARS-CoV-2 diagnostic kit(PathoDetect) which is cheap and as well as faster also the company partnered with Serum Institute of India and APG to scale up the COVID-19 detection kits [25]. Council for Scientific and Industrial Research (CSIR) has partnered with some companies which includes TCS, BHEL and CIPLA for COVID-19 research and development [26]. By employing Immunoinformatics based approach one research group of Gauhati University reported B cell and T cell epitopes in surface glycoprotein of SARS-CoV-2, among which some epitopes can be the potential lead for vaccine against SARS-CoV-2 [27]. Serum Institute of India (SII) in partnership with Codagenix, an American biotechnology firm is likely to be ready with vaccine against SARS-CoV-2 by 2022. The vaccine candidate is a deoptimized live attenuated virus and has progressed to the pre clinical test phase. [23, 28].
References:
1.Coronaviridae—Positive Sense RNA Viruses—Positive Sense RNA Viruses. 2011. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/positive-sense-rna-viruses-2011/w/posrna_viruses/222/coronaviridae
2.Firas A.R., Mazhar S. Al Zoubi et al., SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens, 2020 ; 9, 231; doi:10.3390/pathogens9030231
3. Guangdi Li and Erik De Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nature Reviews Drug Discovery 2020; 19: 149-150.
4.De Clercq, E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chemistry: An Asian Journal. 2019; 14: 3962–3968.
5. Zumla, A. et al. Coronaviruses — drug discovery and therapeutic options. Nature Reviews Drug Discovery 2016; 15: 327–347.
7. Wang M. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research 2020, https://doi.org/10.1038/s41422-020-0282-0
8. Holshue, M. L. et al. First case of 2019 novel coronavirus in the United States. New England Journal of Medicine 2020. 382:929-936. https://doi.org/10.1056/ NEJMoa2001191.
9. Tim Smith, Jennifer Bushek, Tony Prosser. COVID-19 Drug Therapy – Potential Options Clinical Drug Information, Elsevier 2020
10. Liu X, Wang XJ. Potential inhibitors for 2019-nCoV coronavirus M protease from clinically proven medicines. Journal of Genetics and Genomics 2020. PMID: 32173287
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. New England Journal of Medicine 2020 Mar 18. PMID: 32187464
12. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical Infectious Disease 2020. PMID: 32150618.
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of
COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents 2020. PMID: 32205204
14. Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. American Journal of Health-System Pharmacy. 2008; 65(15):1413-8. https://doi: 10.2146/ajhp070449.
15. Wang Z et al. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clinical Infectious Diseases, ciaa272, https://doi.org/10.1093/cid/ciaa272
16. Xu X et al. Effect treatment of severe COVID-19 patients with tocilizumab. ChinaXiv.20200300026.v1. http://www.chinaxiv.org/user/download.htm?id=30387
17. Jin Y., Cai, L., Cheng, Z. et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Military Medicine Research 2020; 7, 4.
18. Regeneron Pharmaceuticals. Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19. Retrieved March 24, 2020. Available on the World Wide Web at: https://clinicaltrials.gov/ct2/show/NCT04315298term=sarilumab&draw=3&rank=.
19. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nature Reviews Microbiology 2004; 2(9): 695–703.
20. Casadevall A, Pirofski L. The convalescent sera option for containing COVID19. The Journal of Clinical Investigation 2020; 130(4): 1545-1548. https://doi.org/10.1172/JCI138003.
21. Shen C, Wang Z, Zhao F et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020. https://doi:10.1001/jama.2020.4783.
23. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1
24. https://www.icmr.nic.in/sites/default/files/MediaReport_COVID19.pdf
25. https://mylabdiscoverysolutions.com/press-release/
27. Baruah V, Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. Journal of Medical Virology 2020; 92(5):495-500. https://doi: 10.1002/jmv.25698.
28. https://www.seruminstitute.com/news.php
Let’s meet Pritam Mukherjee