Novavax vaccine (NVX-CoV2373): Promising Phase-III Human clinical-trial results against Covid-19
Dr. Juni Banerjee, PhD; Neucrad Health Desk, February 20, 2021
Maryland-based NovaVax Biopharma recently developed a protein-based novel coronavirus vaccine. The vaccine is NVX-CoV2373 that has already been able to produce substantial antibodies against the virus in pre-clinical trials. Moreover, the Phase-III clinical trials of the vaccine took place during September in the UK and during December in the US. NovaVax reported that a British clinical trial has proven the vaccine to be 79.3% effective.
Development of NovaVax vaccine (NVX-CoV2373)
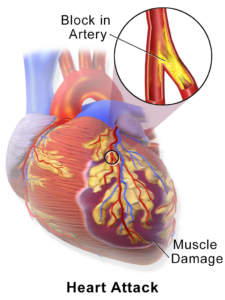
As we know that the SARS-CoV-2 virus is surrounded by spike proteins, which help the virus to attach to the lung cell receptors and enter the cell. Researchers at NovaVax created a modified ‘spike gene’ sequence of the novel coronaviruses and placed it in the middle of the Bacillus virus. Importantly, the Bacillus virus can only infect insects. Henceforth, the researchers choose and infected moths with this recombinant virus. The spike proteins formed in the moth cells, as a result, were purified and attached by means of nanoparticles. This formulation is used as a vaccine.
How does the NovaVax vaccine work?
- The Spike nanoparticle vaccine from Novavax is administrated as an intramuscular injection on upper arms, mixed with a soapbark plant’s purified compound that acts as an adjuvant.
- As a result, the immune cells (lymphocytes, eosinophils, macrophages, etc.) rapidly rush to the vicinity of the injection site and the immune reaction begins.
- Importantly, the antigen-presenting cells uptakes the spike nanoparticle and present it on its cell membrane.
- Next, the antigen-presenting cells present these spike proteins to T-lymphocytes via ‘major histocompatibility complex’ (MHC).
- Under the influence of stimulated T-lymphocytes, the antibody-producing B-lymphocytes becomes activated.
This whole process has two consequences:
- Specific antibodies are produced against the spike protein of SARS-CoV-2 virus.
- Killer T-lymphocytes are formed against some parts of the spike protein of SARS-CoV-2 virus.
The Clinical trials for NovaVax vaccine
NVX-CoV2373 was tested on a total of 15,000 people in a Phase-III clinical trial. About 28% of the selected group were over 65 years of age. The vaccine has shown quite promising results. Additionally, this vaccine even has the ability to counteract the newly discovered variant of the coronavirus of Britain. According to research, NovaVax Vaccine is 8% effective in preventing the new variants. Of note, the Novavax vaccine (which does not contain HIV) is 80% resistant to the South African variant of coronavirus.
NovaVax Vaccine Dosage and Usage
Two doses are given for the NovaVax vaccine. The second dose is given 21 days after the first dose of NovaVax Spike Nanoparticle Vaccine. Scientists are claiming that the effects of this spike nanoparticle vaccine of NovaVax last for many years and hence, can prevent future novel coronavirus infections. Moreover, similar to Pfizer and Modern vaccines, the NovaVax vaccines are easy to store at 4 degrees C for a long time without freezing. Henceforth, the vaccine can be quickly transported to different parts of the country for fighting Covid-19 pandemic.

References:
- Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, Logue J, Portnoff AD, Norton J, Guebre-Xabier M, Zhou B, Jacobson K, Maciejewski S, Khatoon R, Wisniewska M, Moffitt W, Kluepfel-Stahl S, Ekechukwu B, Papin J, Boddapati S, Jason Wong C, Piedra PA, Frieman MB, Massare MJ, Fries L, Bengtsson KL, Stertman L, Ellingsworth L, Glenn G, Smith G. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021 Jan 14;12(1):372. doi: 10.1038/s41467-020-20653-8. PMID: 33446655; PMCID: PMC7809486.
- Keech C, Albert G, Cho I, et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383(24):2320-2332. doi:10.1056/NEJMoa2026920
- Kaur SP, Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020;288:198114. doi:10.1016/j.virusres.2020.198114
- Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano. 2020;14(10):12522-12537. doi:10.1021/acsnano.0c07197
- Pollet J, Chen WH, Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics [published online ahead of print, 2021 Jan 7]. Adv Drug Deliv Rev. 2021;170:71-82. doi:10.1016/j.addr.2021.01.001