Kesimpta®(Ofatumumab): Targeted B-cell Therapy for Relapsing forms of Multiple Sclerosis

Dr. Juni Banerjee, PhD; Neucrad Health Desk, September 4, 2020
The Novartis drug Kesimpta® has been recently approved by FDA to be used for treating Relapsing-forms of Multiple Sclerosis (RMS). Multiple Sclerosis (MS) is a complex, Autoimmune Neurological disease that has affected around 2.3 million people all over the world and is incurable. Even with the advancement of modern Science and Medicine, there are limited numbers of drugs or specific treatment options for MS. Recently, Novartis International AG, the Swiss pharmaceutical company has come up with a breakthrough B-cell targeted therapy for relapsing MS. The name of their invented therapy is “Kesimpta®” (Ofatumumab) which is already an approved drug by The US Food and Drug Administration (FDA) in 2009 for the treatment of Chronic Lymphocytic Leukemia. Before we go into the detail about this Therapy, let’s first comprehend Multiple Sclerosis, its Types, Cause, Diagnosis and Treatment.
Multiple sclerosis:
In case of the disease Multiple Sclerosis, chronic inflammation in the Brain, spinal cord, optic nerve and periventricular spaces happens. The main characteristic feature of MS is Myelin sheath destruction and axonal damage in the brain, spinal cord and optic nerves. MS led to several neurological disorders affecting patient’s normal walking, talking and daily activities. The patient may face paralysis and even death, if left untreated.
Multiple Sclerosis Types:
There are four types of Multiple Sclerosis:
1. Primary Progressive MS: In this MS type, the symptoms slowly increase over time and less harmful to the patients.
2. Secondary Progressive MS: The symptoms of this MS type are quite severe and fast worsening in nature.
3. Progressive-Relapsing MS: In this MS type, the symptoms come back frequently and the severity of the disease is extremely high. However, this condition is rare.
4. Relapsing MS (RMS): This MS type is the most common. Symptoms disappear for a while after the disease has onset. But the symptoms re-appear again after a certain period of time making the patient ill.
Causes of Multiple Sclerosis:
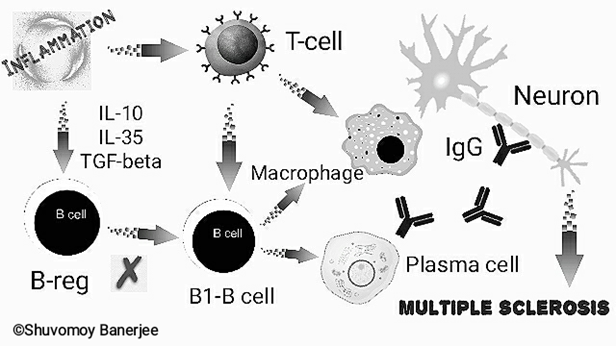
Our body’s immune system fights against pathogens and foreign bodies. But in case of autoimmune diseases body’s own immune cells (e.g. B-lymphocytes etc.) and antibodies (called auto-antibodies) destroy the cells of vital organs. Of note, in case of MS, the neurons of the brain and spinal cord are constantly attacked by plasma-cell generated antibodies, T-lymphocytes and macrophages leading to gradual damage of axonal myelin sheath of those neurons. As a result, communication and function between various nerves is severely hampered.
Although, the exact causes of MS are not fully known, scientists claim that genetic factors, lack of vitamin-D, or pathogenic infections can cause MS. There are several theories behind the molecular mechanisms responsible for MS. The most accepted one is deregulated function of B-lymphocytes!
Humans have 2 main types (sub-sets) of B-lymphocytes:
- B1B and B2B – Studies have shown that B1B cells are particularly responsible for MS.
- B-reg (regulatory B-lymphocytes)- B-reg regulates the function of B1B-lymphocytes or is considered to reduce the activity of B1B-lymphocytes. Increased levels of pro-inflammatory mediators including, IL-10, IL-35, TGF-beta continue to deregulate the functions of B-reg.
Research revealed that the blood levels of pro-inflammatory cytokines are significantly increased in case of MS, resulting in overactive B1B lymphocytes. At this stage, B1B lymphocytes rapidly produce large amounts of immunoglobulins (IgG-antibodies) by plasma cells which get released into the blood and Cerebrospinal Fluid (CSF). Consequently, the over-activated B1B lymphocytes stimulate macrophages, Th1 and Th17 T-lymphocyte responses. Overall, extensive damage to neurons and its myelin sheath occurs.
Treatment of Multiple Sclerosis:
According to the types of MS, the treatment strategy is decided by physicians. Management of relapsing MS is particularly difficult. Usually, anti-inflammatory drugs and steroids are given to reduce the symptoms. If the steroid does not work, plasma exchange or “Plasmapheresis” is performed by injecting a mixture of fresh plasma along with immunoglobulin into the patient’s body. In case of the most common types of MS i.e. Relapsing Multiple Sclerosis (RMS), “Disease Modifying Therapy (DMT)” had been used. In this therapy the patient is treated using Interferon-beta and other approved drugs. However, so far the effectiveness of this treatment and the specificity of such drugs are quite limited.
What’s interesting about Kesimpta®?
- Scientists from Novartis completed the 3rd phase of clinical trials for Kesimpta® (Ofatumumab) therapy in a joint venture with Dr. Stephen L. Hauser of the UCSF Weill Institute for Neuroscience (California, USA) and Dr. Kappos of the University Hospital Basel. Their clinical trials are named ASCLEPIOS-I and II. The Novartis drug Kesimpta® has been recently approved by FDA to be used for treating Relapsing-forms of Multiple Sclerosis (RMS).
- A comparative test of the two different drugs was performed for the clinical trials on RMS patients. Total 946 patients were subcutaneously injected Kesimpta® (20 mg) and 936 other patients were orally given Teriflunomide tablets (14 mg). Both the groups were monitored at regular intervals by MRI scans. Kesimpta® delivered powerful efficacy, significant decrease in risk of relapses, less brain lesions, and probability of halting new disease activity within 1 & 2 years. However, Teriflunomide did not show any significant efficacy.
- Kesimpta® is actually an anti-CD-20 antibody. The CD-20 protein is expressed in cell membrane of active B-lymphocytes that regulates various cellular functions. The most important of these is the rapid cell division of B-lymphocytes and the activation of T-lymphocytes as well. These 2 functions are held responsible for damaging the myelin sheath of the nerve cells in MS. The application of anti-CD-20 antibodies blocks the molecular signaling pathway of the CD-20 protein and reduces the activity of Th1 and Th17 T-lymphocyte response, henceforth, decreasing the overall damage to nerves and myelin sheaths.
- Dr. Krishnan Ramanathan, Director of the Novartis Neuroscience Global Program, confirmed that Kesimpta® is far ahead of other MS drugs in terms of safety, superior efficacy, costing. Only 10% of all patients showed very slight side effects. The most important thing about Kesimpta® is that it is kinda first ever B-cell therapy which is not only precisely-dosed but also can be self-administrated by anyone sitting at home after Physicians’ approval!



Please contact [email protected] for advertisement.
References:
- https://www.novartis.com/search-results?query=ofatumumab
- Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N Engl J Med. 2020;383(6):546-557. doi:10.1056/NEJMoa1917246
- Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(10):977-986. doi:10.1016/S1474-4422(14)70191-7
- Kish T. Promising Multiple Sclerosis Agents In Late-Stage Development. P T. 2018;43(12):750-772.
- Greenfield AL, Hauser SL. B-cell Therapy for Multiple Sclerosis: Entering an era. Ann Neurol. 2018;83(1):13-26. doi:10.1002/ana.25119
- Milo R. Therapies for multiple sclerosis targeting B cells. Croat Med J. 2019;60(2):87-98. doi:10.3325/cmj.2019.60.87
- https://www.nature.com/articles/d42859-018-00030-8
- Arneth BM. Impact of B cells to the pathophysiology of multiple sclerosis. J Neuroinflammation. 2019;16(1):128. Published 2019 Jun 25. doi:10.1186/s12974-019-1517-1
- Racke MK. The role of B cells in multiple sclerosis: rationale for B-cell-targeted therapies. Curr Opin Neurol. 2008;21 Suppl 1:S9-S18. doi:10.1097/01.wco.0000313359.61176.15






